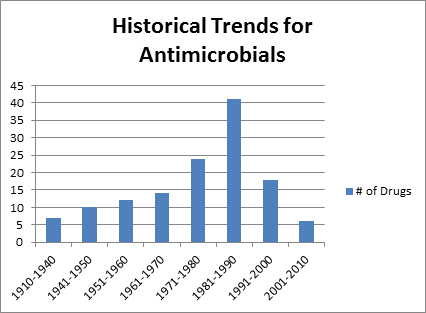
4. Antibiotic Development Pipeline
|
Table of Contents
ACTIVITY OVERVIEW INTRODUCTION SECTION ONE: The global threat of AMDR SECTION TWO: Understanding AMDR 1. Etiology and Epidemiology 2. Incidence and Prevalence of Microbial Resistance 3. Major AMDR Pathogens a. Acinetobacter baumanii b. Clostridium difficile c. Escherichia coli d. HIV/AIDS and Sexually Transmitted Infection e. Influenza virus f. Malaria (Plasmodium) g. Methicillin-resistant Staphylococcus aureus (MRSA) h. Streptococcus pneumoniae i. Tuberculosis and MDR-TB j. Vancomycin-Resistant Enterococcus (VRE) SECTION THREE: Control and Prevention of AMDR 1. Implications of Microbial Resistance 2. Infections and Chronic Diseases 3. Policies and Best Practices a. Antimicrobial Drug Stewardship b. Surveillance c. Environmental Decontamination d. Infection Control e. Patient Education 4. Antibiotic Development Pipeline SECTION FOUR: Conclusions REFERENCES APPENDICES GLOSSARY Test Questions Program Evaluation Self Assessment |


|